The chapter “Structure of Atom” in Class 9 Science explains how atoms are made up of smaller particles like electrons, protons, and neutrons. It also discusses important models of the atom proposed by scientists such as J.J. Thomson, Rutherford, and Bohr, along with concepts like atomic number, mass number, and valency.
Structure of Atom Class 9 Notes
Charged Particles in Matter
When you rub two things together, like a comb on dry hair or a glass rod with silk, they can attract other objects. This happens because rubbing moves tiny particles called electrons from one object to another, giving them an electric charge. Atoms, which make up everything around us, contain even smaller parts—electrons (negative charge) and protons (positive charge). Scientists like J.J. Thomson and E. Goldstein discovered these particles. Electrons are light and can move easily, while protons are heavier and stay inside the atom. These particles balance each other to keep the atom stable.
The Structure of an Atom
The two fundamental particles (electrons and protons) inside the atom led to the failure of this aspect of Dalton’s atomic theory. It was then considered necessary to know how electrons and protons are arranged within an atom. For explaining this, many scientists proposed various atomic models. J.J. Thomson was the first one to propose a model for the structure of an atom.
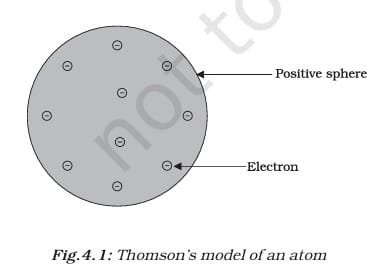
THOMSON’S MODEL OF AN ATOM
Thomson proposed that an atom looks like a Christmas pudding or a watermelon. In his model, the atom is a positively charged sphere with negatively charged electrons embedded in it—like dry fruits in a pudding or seeds in a watermelon. He said the positive and negative charges are equal, so the atom is neutral overall. While this model explained electrical neutrality, it couldn’t explain results from later experiments, which led scientists to search for a better atomic model.
Thomson proposed that:
- An atom consists of a positively charged sphere, and the electrons are embedded in it.
- The negative and positive charges are equal in magnitude. So, the atom as a whole is electrically neutral.
RUTHERFORD’S MODEL OF AN ATOM
Ernest Rutherford wants to know how the electrons are arranged in an atom. He did one experiment where one person shot fast-moving alpha particles at a very thin gold foil. He expected small deflections, but the result surprised him:
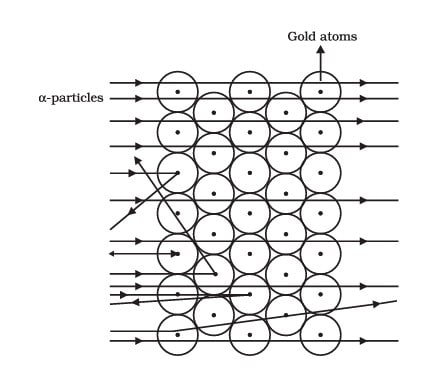
- Most of the space inside the atom is empty because most of the α-particles passed through the gold foil without getting deflected.
- Very few particles were deflected from their path, indicating that the positive charge of the atom occupies very little space.
- A very small fraction of α-particles were deflected by 1800, indicating that all the positive charge and mass of the gold atom were concentrated in a very small volume within the atom.
This showed that most of the atom is empty space and the positive charge and mass are concentrated in a tiny central part, which Rutherford called the nucleus.
Key Features of Rutherford’s Model:
- An atom has a small, dense, positively charged nucleus.
- Electrons revolve around the nucleus in circular paths.
- The nucleus is tiny compared to the whole atom.
Drawback:
According to physics, moving electrons should lose energy and spiral into the nucleus, making atoms unstable. But atoms are stable, so this model couldn’t fully explain atomic structure.
BOHR’S MODEL OF THE ATOM
Bohr’s Model of the Atom is a description of the atomic structure proposed by Danish physicist Niels Bohr in 1913. According to the model, atoms consist of a central nucleus, made up of protons and neutrons, surrounded by electrons that are in orbit around the nucleus.
- Neils Bohr proposed that only certain, specific orbits for electrons within an atom are permitted and are referred to as “discrete orbits”.
- According to Bohr’s model, electrons in these orbits do not emit energy while they revolve. These orbits, or shells, are referred to as energy levels and are designated by letters (K, L, M, N, etc.) or numbers (n=1, 2, 3, 4, etc.).
These orbits or shells are called energy levels. Energy levels in an atom are shown in
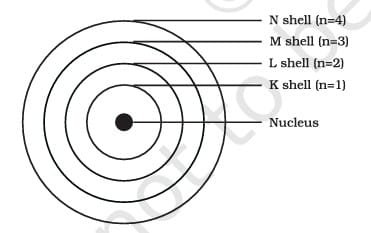
These orbits or shells are represented by the letters K, L, M, N,… or the numbers n=1, 2, 3, 4,….
NEUTRONS
In 1932, J. Chadwick discovered another subatomic particle, which had no charge and a mass nearly equal to that of a proton. It was eventually named as neutron. Neutrons are present in the nucleus of all atoms, except hydrogen. In general, a neutron is represented as ‘n.’. The mass of an atom is therefore given by the sum of the masses of protons and neutrons present in the nucleus.
How are electrons distributed in different orbits (shells)?
The distribution of electrons into different orbits of an atom was suggested by Bohr and Bury. The following rules are followed for writing the number of electrons in different energy levels or shells:
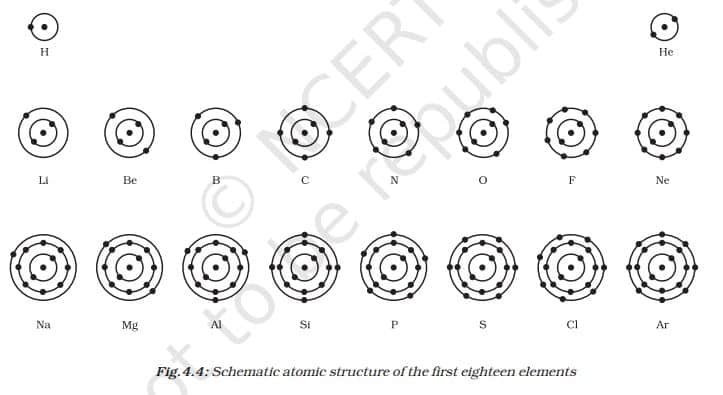
(i) The maximum number of electrons present in a shell is given by the formula 2n², where ‘n’ is the orbit number or energy level index, 1, 2, 3,….
Hence, the maximum number of electrons in different shells are as follows:
- The first orbit, or K-shell, will be 2 × 12 = 2.
- second orbit, or L-shell, will be 2 × 22 = 8,
- The third orbit, or M-shell, will be 2 × 32 = 18.
- The fourth orbit, or N-shell, will be 2 × 42.
- = 32, and so on.
(ii) The maximum number of electrons that can be accommodated in the outermost orbit is 8.
(iii) Electrons are not accommodated in a given shell unless the inner shells are filled. That is, the shells are filled in a stepwise manner.
Valency
Valency is the combining capacity of an atom, which depends on the number of electrons in its outermost shell (called valence electrons). Atoms try to achieve a full outer shell—usually 8 electrons, known as an octet—by gaining, losing, or sharing electrons.
- If an atom has 1, 2, or 3 electrons, it tends to lose them → valency equals the number lost.
- If it has 5, 6, or 7 electrons, it tends to gain electrons to complete 8 → valency equals 8 minus the number of electrons.
- Atoms with full outer shells (like helium or neon) are inert and have a valency of 0.
Atomic Number and Mass Number
Atomic Number
A unique number of protons in the nucleus of an atom is known as an atomic number indicates how many protons make up an atom’s nucleus. An element’s identification and place on the periodic table are both determined by this number. Known also as the proton number, the atomic number is denoted by the letter “Z.”
Mass number
The total number of protons and neutrons in an element’s atoms determines its mass number. “A” symbol referes to a mass number.
Isotopes
In nature, a number of atoms of some elements have been identified, which have the same atomic number but different mass numbers. For example, take the case of hydrogen atom, it has three atomic species, namely protium (1/1 H), deuterium ( 2/1 H or D) and tritium ( 3/1 H or T). The atomic number of each one is 1, but the mass number is 1, 2 and 3, respectively.
Applications
Since the chemical properties of all the isotopes of an element are the same, normally we are not concerned about taking a mixture. But some isotopes have special properties which find them useful in various fields. Some of them are :
- An isotope of uranium is used as a fuel in nuclear reactors.
- An isotope of cobalt is used in the treatment of cancer.
- An isotope of iodine is used in the treatment of goitre.
ISOBARS
Isobars are atoms with the same mass number but different atomic numbers, in other words, they are atoms of different elements that have the same atomic mass.
Disclaimer: We have taken an effort to provide you with the accurate handout of “Structure of Atom Class 9 Notes“. If you feel that there is any error or mistake, please contact me at anuraganand2017@gmail.com.
The above CBSE study material present on our websites is for education purpose, not our copyrights. All the above content and Screenshot are taken from Science Class 9 NCERT Textbook, CBSE Sample Paper, CBSE Old Sample Paper, CBSE Board Paper and CBSE Support Material which is present in CBSEACADEMIC website, NCERT websiteThis Textbook and Support Material are legally copyright by Central Board of Secondary Education. We are only providing a medium and helping the students to improve the performances in the examination.
Images and content shown above are the property of individual organizations and are used here for reference purposes only.
For more information, refer to the official CBSE textbooks available at cbseacademic.nic.in
