Structure of Atom Class 9 Notes – By 1900, scientists knew atoms contained electrons (discovered by J.J. Thomson) and protons (discovered through canal rays discovered by E. Goldstein). Protons have a mass 2000 times greater and a charge equal in magnitude but opposite to that of electrons. Atoms are composed of balancing protons and electrons, with protons in the interior. The structure of these sub-atomic particles within the atom was a question that remained.
Structure of Atom Class 9 Notes
The Structure of an Atom
Thomson’s Model of an Atom
In 1904, J.J. Thomson’s model of an atom is introduce, This model is also known as ‘plum pudding model’. According to this model –
- The atom was considered to be a positively charged mass with negatively charged electrons “embedded” within it.
- The electrons were believed to be spread evenly throughout the atom, causing it to be neutral overall.
- The electrons were held in their positions by an attractive force between the negatively charged electrons and the positively charged mass.

Rutherford’s model of an Atom
In 1911, Rutherford’s model of an atom is introduce, This model is also known as ‘nuclear model’ or ‘planetary model’. Based on the results of Rutherford’s famous gold foil experiment. According to this model –
- The majority of an atom’s mass and positive charge is concentrated in a small, dense nucleus at the center of the atom.
- The electrons are located in shells surrounding the nucleus and are held in their positions by electromagnetic attraction to the positively charged nucleus.
- The Rutherford model explained the results of the gold foil experiment, in which some alpha particles were deflected by large angles.
- The Rutherford model established the basic structure of the atom, with a central nucleus surrounded by orbiting electrons.
- It is still considered to be an important contribution to our understanding of atomic structure.

The alpha particle scattering experiment produced unexpected findings. The following things were noticed:
- The majority of fast-moving alpha particles passed straight through the gold foil.
- The foil caused minor angular deflections in some alpha particles.
- Unexpectedly, about one out of every 12,000 particles appeared to bounce back.
Rutherford’s conclusions from his observations
- The majority of space inside the atom is empty because alpha particles passed through the gold foil.
- A small number of particles were deflected from their path, as the positive charge of the atom occupies only a small amount of space.
- A tiny fraction of alpha particles rebounded back, indicating that all the positive charge and mass of the gold atom is concentrated in a very small volume within the atom.
- The calculated radius of the nucleus was 105 times smaller than the radius of the atom.
Nuclear Model of an Atom
- The center of the atom is comprised of positive charge, referred to as the nucleus. The atom’s nucleus is where the majority of its mass is located.
- The electrons in an atom are located outside the nucleus and revolve around it in orbits.
- The size of the nucleus is much smaller compared to the size of the entire atom.
Drawbacks of Rutherford’s model of the atom
- Rutherford proposed that electrons in an atom revolve around the nucleus in well-defined orbits.
- However, as particles in a circular orbit would experience acceleration, the revolving electrons would lose energy and eventually fall into the nucleus.
- This would result in the atom being highly unstable and matter would not exist in the form that we observe.
- This inconsistency with observed stability led to the need for further developments in atomic theory.
Bohr’s Model of Atom
Bohr’s Model of the Atom is a description of the atomic structure proposed by Danish physicist Niels Bohr in 1913. According to the model, atoms consist of a central nucleus, made up of protons and neutrons, surrounded by electrons that are in orbit around the nucleus.
- Neils Bohr proposed that only certain, specific orbits for electrons within an atom are permitted and are referred to as “discrete orbits”.
- According to Bohr’s model, electrons in these orbits do not emit energy while they revolve. These orbits, or shells, are referred to as energy levels and are designated by letters (K, L, M, N, etc.) or numbers (n=1, 2, 3, 4, etc.).

Neutrons
Bohr and Bury’s rules for the distribution of electrons in different orbits (shells) are as follows:
- The maximum number of electrons in a shell is determined by the equation 2n2, where n is the number of the shell. For example, if n = 1, then the maximum number of electrons in the first shell (K shell) is 2 * 12 = 2 electrons.
- The outermost shell can accommodate a maximum of 8 electrons.
- Electrons fill shells in a stepwise manner, starting from the innermost shell and moving outward. A given shell will not be filled until all the inner shells are completely filled.
Valency
- Valence electrons are the ones that make up an atom’s outermost shell.
- An atom’s tendency to react and form molecules with other atoms is known as its valency or combining capacity.
- Atoms with a completely filled outermost shell have low chemical activity and a valency of zero.
- The quantity of electrons in an atom’s outermost shell determines its valency. For example, hydrogen has one valence electron, making its valency 1, while magnesium has two valence electrons, making its valency 2.
- An atom can attain stability by losing its valence electrons, and the number of electrons it can lose is equal to its valency. For example, hydrogen can easily lose 1 electron to achieve stability, while magnesium can lose 2 electrons.
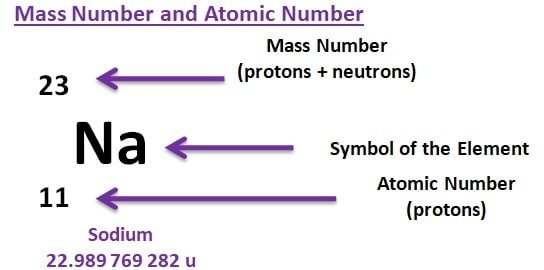
Atomic Number
A unique number of protons in the nucleus of an atom is known as an atomic number indicates how many protons make up an atom’s nucleus. An element’s identification and place on the periodic table are both determined by this number. Known also as the proton number, the atomic number is denoted by the letter “Z.”
Mass number
The total number of protons and neutrons in an element’s atoms determines its mass number. “A” symbol referes to a mass number.
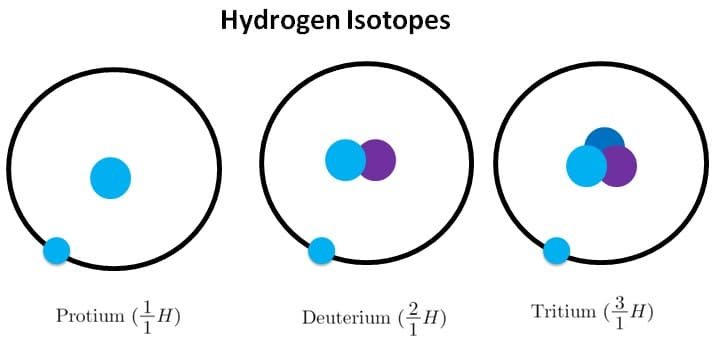
Isotopes
Isotopes are atoms of the same element with the same atomic number but with a different mass number.
Applications of isotopes
- A specific isotope of Uranium, typically U-235, is used as fuel in nuclear reactors.
- In cancer treatment, a particular isotope of Cobalt, usually Co-60, is utilized.
- A certain isotope of Iodine, usually I-131, is employed in the treatment of goitre, which is an enlargement of the thyroid gland.
Isobars
Isobars are atoms with the same mass number but different atomic numbers, in other words, they are atoms of different elements that have the same atomic mass.

