Is Matter Around Us Pure Class 9 Notes – In this chapter, students learn about the concept of pure substances and mixtures, their types, and separation techniques used in everyday life. These notes provide a clear explanation of elements, compounds, and mixtures with examples, along with easy definitions.
Matter Around us Pure Class 9 Notes
What is a Mixture?
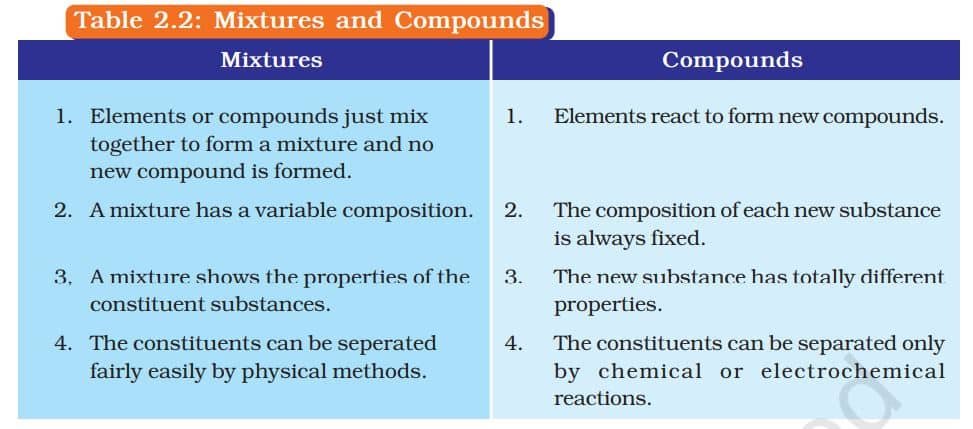
Mixtures are constituted by more than one kind of pure form of matter. We know that dissolved sodium chloride can be separated from water by the physical process of evaporation. However, sodium chloride is itself a pure substance and cannot be separated by physical process into its chemical constituents. Similarly, sugar is a substance which contains only one kind of pure matter and its composition is the same throughout.
TYPES OF MIXTURES
Depending upon the nature of the components that form a mixture, we can have different types of mixtures.
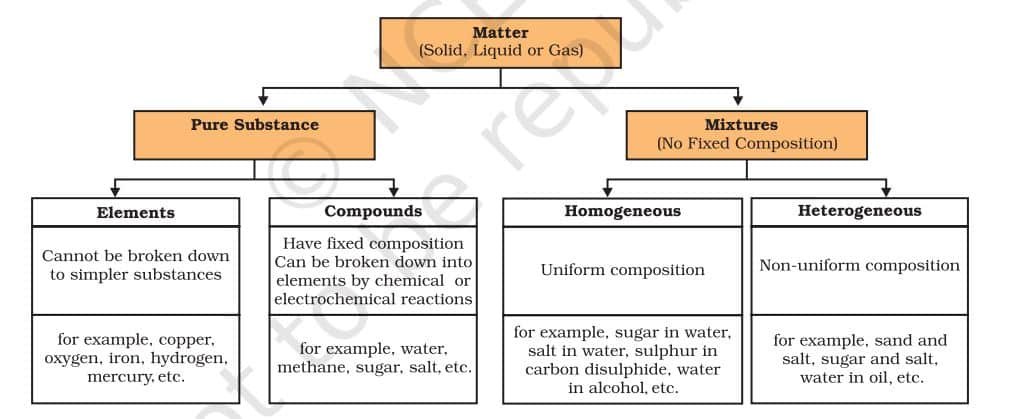
1. Homogeneous Mixtures (Solutions)
These mixtures have a uniform composition throughout, meaning the individual components are not visibly distinguishable.
Characteristics:
- Appears as a single phase (solid, liquid, or gas)
- Components are evenly distributed
- Difficult to separate by simple physical means
Examples:
- Salt dissolved in water
- Air (a mixture of gases)
- Vinegar
- Alloys like brass (copper + zinc)
2. Heterogeneous Mixtures
These mixtures have a non-uniform composition, and the different components are visibly distinct.
Characteristics:
- May consist of multiple phases
- Components are not evenly mixed
- Easier to separate using physical methods
Examples:
- Salad (vegetables mixed together)
- Sand and iron filings
- Oil and water
- Granite
Comparison Table
| Feature | Homogeneous Mixture | Heterogeneous Mixture |
|---|---|---|
| Composition | Uniform | Non-uniform |
| Visibility of components | Not visible | Clearly visible |
| Separation method | More complex | Easier (filtration, etc.) |
| Examples | Saltwater, air, alloys | Salad, soil, oil-water |
What is a Solution?
A solution is a homogeneous mixture of two or more substances. You come across various types of solutions in your daily life. Lemonade, soda water, etc., are all examples of solutions.
Properties of a Solution
- A solution is a homogeneous mixture.
- The particles of a solution are smaller than 1 nm (10-9 metre) in diameter. So, they cannot be seen by naked eyes.
- Because of very small particle size, they do not scatter a beam of light passing through the solution. So, the path of light is not visible in a solution.
- The solute particles cannot be separated from the mixture by the process of filtration. The solute particles do not settle down when left undisturbed, that is, a solution is stable.
CONCENTRATION OF A SOLUTION
A solution is a mixture of solute and solvent. Its concentration depends on how much solute is present. If there’s less solute, it’s called a dilute solution; if there’s more, it’s concentrated. When no more solute can dissolve at a given temperature, the solution is saturated. If more solute can still dissolve, it’s unsaturated. There are various ways of expressing the concentration of a solution, but here we will learn only three methods.

WHAT IS A SUSPENSION?
A suspension is a type of mixture where solid particles are spread out in a liquid but do not dissolve. These particles stay floating for some time and can be seen with the naked eye. Over time, they settle down if left undisturbed. This kind of mixture is called heterogeneous because the solute and solvent are not evenly mixed.
Properties of a Suspension
- Suspension is a heterogeneous mixture.
- The particles of a suspension can be seen by the naked eye.
- The particles of a suspension scatter a beam of light passing through it and make its path visible.
- The solute particles settle down when a suspension is left undisturbed, that is, a suspension is unstable. They can be separated from the mixture by the process of filtration. When the particles settle down, the suspension breaks and it does not scatter light any more.
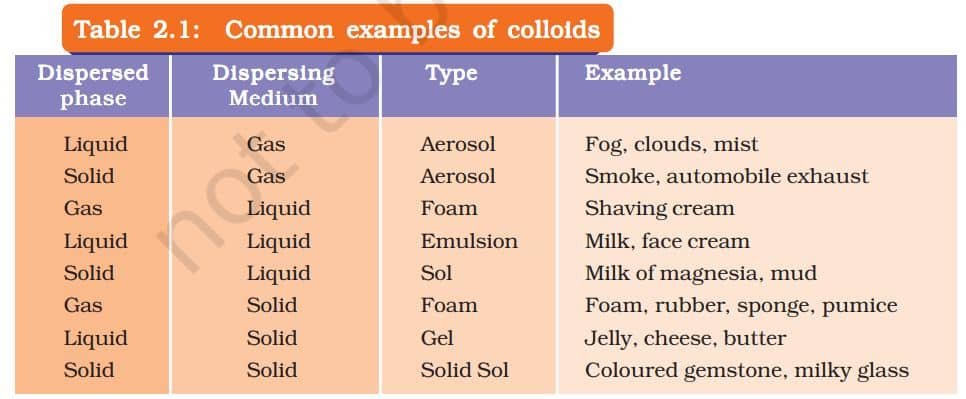
WHAT IS A COLLOIDAL SOLUTION?
A colloidal solution is a special type of mixture where very tiny particles are spread evenly throughout a liquid. These particles are smaller than those in a suspension, so the mixture looks uniform, but it’s actually a heterogeneous mixture. A common example is milk. The particles in a colloid are too small to see with the naked eye, but they can scatter light. This scattering of light is called the Tyndall effect. You can see this effect when sunlight passes through mist in a forest or when a beam of light enters a dusty room.
Properties of a Colloid
- A colloid is a heterogeneous mixture.
- The size of particles of a colloid is too small to be individually seen with naked eyes.
- Colloids are big enough to scatter a beam of light passing through it and make its path visible.
- They do not settle down when left undisturbed, that is, a colloid is quite stable.
- They cannot be separated from the mixture by the process of filtration. But, a special technique of separation known as centrifugation can be used to separate the colloidal particles.
Physical and Chemical Changes
Physical changes: Physical changes involve a change in a substance’s physical properties, such as size, shape, or state (solid, liquid, or gas), without any change to its chemical composition. Examples of physical changes include melting, freezing, cutting, and grinding.
Chemical changes: Chemical transformations are processes that result in new compounds with properties and composition that differ from the original substances. A release or absorption of energy, such as heat, light, or sound, usually occurs. The chemical processes of burning, rusting, and digestion are a few examples.
What are the Types of Pure Substances?
On the basis of their chemical composition, substances can be classified either as elements or compounds.
ELEMENTS
Robert Boyle was the first scientist to use the term element in 1661. Antoine Laurent Lavoisier (1743–94), a French chemist, was the first to establish an experimentally useful definition of an element. He defined an element as a basic form of matter that cannot be broken down into simpler substances by chemical reactions. Elements can be normally divided into metals, non-metals and metalloids.
Metals usually show some or all of the following properties:
- They have a lustre (shine).
- They have silvery-grey or golden-yellow colour.
- They conduct heat and electricity.
- They are ductile (can be drawn into wires).
- They are malleable (can be hammered into thin sheets).
- They are sonorous (make a ringing sound when hit).
Examples of metals are gold, silver, copper, iron, sodium, potassium etc. Mercury is the only metal that is liquid at room temperature.
Non-metals usually show some or all of the following properties:
- They display a variety of colours.
- They are poor conductors of heat and electricity.
- They are not lustrous, sonorous or malleable.
Examples of non-metals are hydrogen, oxygen, iodine, carbon (coal, coke), bromine, chlorine etc.
COMPOUNDS
A compound is a substance made when two or more elements combine chemically in a fixed ratio. For example, when iron and sulphur are just mixed (Group I), they form a mixture—each element keeps its own properties. But when the same mixture is heated (Group II), a chemical reaction takes place, forming a new compound called iron sulphide, which has completely different properties. This shows that a compound is not just a mix—it’s a new substance with new characteristics. Unlike mixtures, compounds cannot be separated by simple physical methods.
Disclaimer: We have taken an effort to provide you with the accurate handout of “Matter in our Surroundings Class 9 Notes“. If you feel that there is any error or mistake, please contact me at anuraganand2017@gmail.com.
The above CBSE study material present on our websites is for education purpose, not our copyrights. All the above content and Screenshot are taken from Science Class 9 NCERT Textbook, CBSE Sample Paper, CBSE Old Sample Paper, CBSE Board Paper and CBSE Support Material which is present in CBSEACADEMIC website, NCERT websiteThis Textbook and Support Material are legally copyright by Central Board of Secondary Education. We are only providing a medium and helping the students to improve the performances in the examination.
Images and content shown above are the property of individual organizations and are used here for reference purposes only.
For more information, refer to the official CBSE textbooks available at cbseacademic.nic.in
