Science Class 10 MCQ Sample Paper 1 is designed to help students prepare effectively for their CBSE Board Exams. This practice set includes chapter-wise and mixed Multiple Choice Questions based on the latest CBSE syllabus and exam pattern. These MCQs help students improve conceptual understanding, boost accuracy, and strengthen exam readiness. Solve these questions regularly to build confidence and score higher marks in Class 10 Science.
Science Class 10 MCQ Sample Paper 1
Question 1: Select the group in which all organisms have the same mode of nutrition.
a. Cuscuta, yeast, legumes, leeches and tapeworm
b. Cactus, ticks, lice, leeches and cow
c. Cuscuta, ticks, lice, leeches and tapeworm
d. Cactus, grass, lice, lion and tapeworm
Question 2: Which of the following options indicates the products formed after breakdown of the glucose in our muscle cells when there is lack of oxygen?
a. Ethanol + carbon dioxide + Energy
b. Lactic acid + Energy
c. Lactic acid + carbon monoxide + Energy
d. Carbon dioxide + Water + Energy
Question 3: Which of the following is a correct combination of function and part of the brain?
a. Posture and balance: Cerebrum
b. Salivation: Medulla in midbrain
c. Hunger: Pons in hindbrain
d. Blood pressure: Medulla in hindbrain
Question 4: The blood glucose level in a patient was very high. It may be due to inadequate secretion of:
a. growth hormone from pituitary gland
b. oestrogen from ovary
c. insulin from pituitary gland
d. insulin from pancreas
Question 5: In a cross between black furred rabbit (B) and white furred rabbit (b), all offspring were found to have black fur. What can be inferred about the genetic makeup of the parent rabbits?
a. BB X bb
b. Bb X Bb
c. Bb X bb
d. bb X bb
Question 6: Which are the correct statements related to ozone?
(i) Ozone layer helps in increasing the UV radiations reaching earth.
(ii) Ozone is a deadly poison.
(iii) Ozone layer shields the earth from UV radiations.
(iv) Ozone layer prevents UV rays which cause skin cancer.
(v) Ozone is formed with the help of Chloroflurocarbons.
a. (i), (ii), (iii)
b. (ii), (iii), (iv)
c. (iii), (iv), (v)
d. (i), (iv), (v)
Question 7: Which of the following human activities has resulted in an increase of non biodegradable substances?
a. Organic farming
b. Increase in tree plantation
c. Use of plastic as packaging material
d. Composting of kitchen waste
Question 8: Assertion (A): Tallness of a pea plant is controlled by an enzyme.
Reason (R): The gene for that enzyme makes proteins which help the plant to be tall.
a. Both A and R are true, and R is the correct explanation of a.
b. Both A and R are true, and R is not the correct explanation of a.
c. A is true but R is false.
d. A is false but R is true.
Question 9: Assertion (A): Vulture will always have the least amount of pesticides in a food chain.
Reason (R): Vulture occupies the last trophic level and it gets only 10% of energy of the previous trophic level.
a. Both A and R are true, and R is the correct explanation of a.
b. Both A and R are true, and R is not the correct explanation of a.
c. A is true but R is false.
d. A is false but R is true.
Question 10: Which of the following equations represent redox reactions and what are the values for ‘p’ and ‘q’ in these equations?
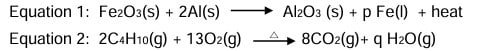
a. Only equation 1 is a redox reaction, p =1 and q=3
b. Both equations 1 and 2 are redox reactions, p= 2 and q=4
c. Only equation 2 is a redox reaction, p= 2 and q= 10
d. Both equations 1 and 2 are redox reactions, p= 2 and q=10
Question 11: Four statements about the reactions of oxides with dilute hydrochloric acid and aqueous sodium hydroxide are listed.
I. Aluminium oxide reacts with both dilute hydrochloric acid and aqueous sodium hydroxide.
II. Calcium oxide reacts with dilute hydrochloric acid and aqueous sodium hydroxide.
III. Zinc oxide reacts with both dilute hydrochloric acid and aqueous sodium hydroxide.
IV. Sulphur dioxide does not react with either dilute hydrochloric acid or aqueous sodium hydroxide.
Which statements are correct?
A. I and II
B. I and III
C. II and IV
D. III and IV
Question 12: An iron nail is added to each of the two test tubes ‘P’ and ‘Q’ containing aqueous copper (II) sulphate, and aqueous silver nitrate respectively. Which of the following observation is correct?
A. In test tube ‘P’ iron nail is coated with a blue coating and in test tube ‘Q’ there is no reaction.
B. Iron nail is coated with a brown coating in test tube ‘P’ and silver coating in test tube ‘Q’.
C. There is no reaction in either of the test tubes ‘P’ or ‘Q’.
D. There is no reaction in test tube ‘P’ but a silver coating on iron nail is seen in test tube ‘Q’.
in test tube ‘Q’.
Question 13: Methyl orange is added to dilute hydrochloric acid and to aqueous sodium hydroxide. What is the colour of the methyl orange in each solution?
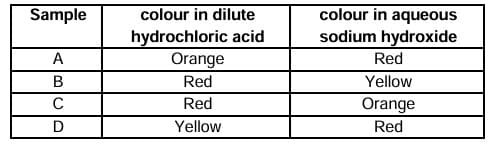
a. Orange Red
b. Red Yellow
c. Red Orange
d. Yellow Red
Question 14: Which of the following substances when dissolved in equal volume of water, will have the highest pH value?
a. Sulphuric acid
b. Acetic acid
c. Magnesium hydroxide
d. Sodium hydroxide
Question 15: When excess of carbon dioxide is passed through lime water, the milkiness disappears because
a. water soluble calcium carbonate converts to water soluble calcium bicarbonate.
b. insoluble calcium carbonate converts to water soluble calcium bicarbonate.
c. water soluble calcium carbonate converts to insoluble calcium bicarbonate.
d. insoluble calcium carbonate converts to insoluble calcium bicarbonate.
Question 16: In the reaction of aqueous solution of barium chloride with aqueous solution of sodium sulphate, the aqueous solution formed will be:
a. BaCl2
b. BaSO4
c. Na2SO4
d. NaCl
Question 17: Assertion (A): C4H8, C4H6 and C4H10 are members of the same homologous series
Reason (R): C4H8, C4H6, C3H4, C3H6, C2H4, C2H2 are unsaturated hydrocarbons.
A. Both A and R are true, and R is the correct explanation of A.
B. Both A and R are true, and R is not the correct explanation of A.
C. A is true but R is false.
D. A is false but R is true.
Question 18: Arnav was making notes and he wrote down the following statements from his understanding of reflection from curved surfaces.
I. Concave mirrors can produce both real and virtual images depending on the position of the object.
II. Convex mirrors always produce real, inverted images regardless of the object’s position.
III. In both concave and convex mirrors, the image location can be determined using the mirror formula 1/𝑓 =(1/ 𝑣) +(1/ 𝑢) where f is the focal length, v is the image distance, and u is the object distance.
Choose from the following the correct option that lists the correct statements about reflection from curved surfaces.
A. I and II
B. I, II and III
C. II and III
D. I and III
Question 19: Choose the correct option from the below which explains the reason for us to perceive the day sky as blue.
A. As sunlight passes through the atmosphere, shorter wavelengths, such as blue are scattered more than other colors.
B. The sky appears blue because all colors are scattered equally, but blue light is stronger and more visible to the human eye.
C. The blue color of the sky is due to longer wavelengths like red and orange scattering more than shorter wavelengths, making blue stand out more.
D. The atmosphere contains blue-colored particles that give the sky its blue appearance.
Question 20:

A. Both A and R are true, and R is the correct explanation of A.
B. Both A and R are true, and R is not the correct explanation of A.
C. A is true but R is false.
D. A is false but R is true.
Question 21:
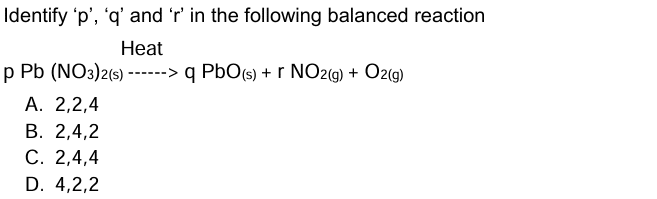
Question 22:
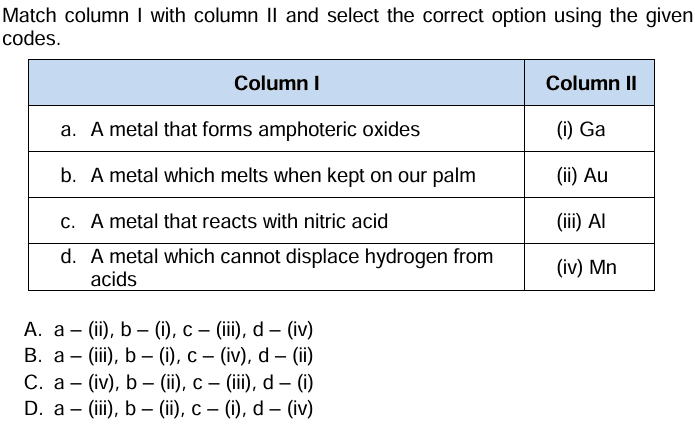
Question 23:
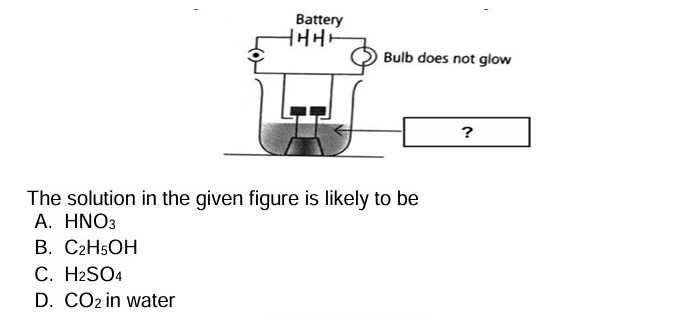
Question 24: An aqueous solution ‘A’ turns the phenolphthalein solution pink. On addition of an aqueous solution ‘B’ to ‘A’, the pink colour disappears. Which of the following statement is true for the solutions ‘A’ and ‘B’.
a. A is strongly basic and B is a weak base.
b. A is strongly acidic and B is a weak acid.
c. A has a pH greater than 7 and B has a pH less than 7.
d. A has a pH less than 7 and B has a pH greater than 7.
Question 25: When 50g of lead powder is added to 300 ml of blue copper sulphate solution, after a few hours, the solution becomes colourless. This is an example of __.
a. Combination reaction
b. Decomposition reaction
c. Displacement reaction
d. Double displacement reaction
Question 26: The electronic configuration of three elements X, Y and Z are X- 2, 8, 7; Y- 2, 8, 2; and Z – 2, 8
a. Y and Z are metals
b. Y and X are non-metals
c. X is a non -metal and Y is a metal
d. Y is a non-metal and Z is a metal
Question 27: Which of the following is an endothermic reaction?
a. Burning of candle.
b. Cooking of food.
c. Decomposition of Vegetable matter.
d. Reaction of Sodium with air
Question 28: During cellular oxidation of Glucose, ATP is produced along with formation of other products in this reaction. Which of the following events is associated with production of maximum ATP molecules per molecule of Glucose during this process? Synthesis of
a. ethanol in yeast
b. lactic acid in muscle cells
c. carbon dioxide in yeast cells
d. carbon dioxide in human cells
Question 29: During which of the following stages of the circulation of blood in a normal human being, the oxygenated blood is pumped to all parts of the body?
a. contraction of the left atrium
b. contraction of left ventricle
c. relaxation of the right atrium
d. relaxation of the right ventricle
Question 30: Which of the following adaptations in herbivores helps in digestions of cellulose?
a. Longer large intestine
b. Smaller large intestine
c. Smaller small intestine
d. Longer small intestine
Question 31: There was a cerebellar dysfunction in a patient. Which of the following activities will get disturbed in this patient as a result of this?
a. Salivation
b. Hunger control
c. Posture and balance
d. Regulation of blood pressure
Question 32: In snails individuals can begin life as male and depending on environmental conditions they can become female as they grow. This is because
a. male snails have dominant genetic makeup.
b. female snails have dominant genetic makeup.
c. expression of sex chromosomes can change in a snail’s life time.
d. sex is not genetically determined in snails.
Question 33: In the following cases, a ray is incident on a concave mirror. In which case is the angle of incidence equal to zero?
a. A ray parallel to the principal axis.
b. A ray passing through the centre of curvature and incident obliquely.
c. A ray passing through the principal focus and incident obliquely.
d. A ray incident obliquely to the principal axis, at the pole of the mirror.
Question 34:
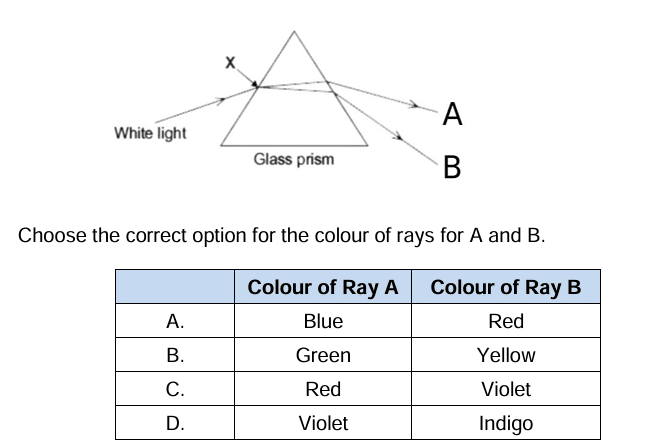
Question 35: Identify the incorrect statement
‘The energy available to the producers is maximum’ because:
a. It is the first trophic level which absorbs1% of light energy directly from the source.
b. It utilizes the most of the chemical energy for its own respiration, growth, reproduction, movement etc.
c. It utilizes 10% of light energy and transfers the rest to the next trophic level.
d. It transfers only 10% of light energy to the next trophic level.
Question 36: Which of the following is not a role of decomposers in the ecosystem?
a. Natural replenishment of soil.
b. Enrichment of oxygen in atmosphere.
c. Waste decomposition.
d. Break-down of dead remains.
Question 37: Assertion (A): On adding dil. HCl to a test tube containing a substance ‘X’, a colourless gas is produced which gives a pop sound when a burning match stick is brought near it.
Reason (R): In this reaction metal ‘X’ is displaced by Hydrogen.
a. Both A and R are true, and R is the correct explanation of a.
b. Both A and R are true, and R is not the correct explanation of a.
c. A is true but R is false.
d. A is false but R is true
Question 38: Assertion (A): Generally, the number of chromosomes in a cell and in a germ cell is not the same in species.
Reason (R): When two germ cells combine, they restore the normal number of chromosomes in a species.
a. Both A and R are true, and R is the correct explanation of a.
b. Both A and R are true, and R is not the correct explanation of a.
c. A is true but R is false.
d. A is false but R is true
Question 39: Assertion (A): A convex mirror always forms an image behind it and the image formed is virtual.
Reason (R): According to the sign convention, the focal length of a convex mirror is positive.
a. Both A and R are true, and R is the correct explanation of a.
b. Both A and R are true, and R is not the correct explanation of a.
c. A is true but R is false.
d. A is false but R is true
Question 40: Assertion (A): If the lions are removed from a food chain it will not affect the food chain, however if the plants are removed from a food chain it will disturb the ecosystem.
Reason (R): Plants are producers who can make food using sunlight, while lions are consumers.
a. Both A and R are true, and R is the correct explanation of a.
b. Both A and R are true, and R is not the correct explanation of a.
c. A is true but R is false.
d. A is false but R is true
Disclaimer: We have provide you with the accurate handout of “Science Class 10 MCQ Sample Paper 1“. If you feel that there is any error or mistake, please contact me at anuraganand2017@gmail.com. The above CBSE study material present on our websites is for education purpose, not our copyrights.
All the above content and Screenshot are taken from Science Class 10 NCERT Textbook, CBSE Sample Paper, CBSE Old Sample Paper, CBSE Board Paper and CBSE Support Material which is present in CBSEACADEMIC website, NCERT website This Textbook and Support Material are legally copyright by Central Board of Secondary Education or NCERT. We are only providing a medium and helping the students to improve the performances in the examination.
Images and content shown above are the property of individual organisations and are used here for reference purposes only. To make it easy to understand, some of the content and images are generated by AI and cross-checked by the teachers. For more information, refer to the official CBSE textbooks available at cbseacademic.nic.in.
