Science Class 10 MCQ Sample Paper 2 is designed to help students prepare effectively for their CBSE Board Exams. This practice set includes chapter-wise and mixed Multiple Choice Questions based on the latest CBSE syllabus and exam pattern. These MCQs help students improve conceptual understanding, boost accuracy, and strengthen exam readiness. Solve these questions regularly to build confidence and score higher marks in Class 10 Science.
Science Class 10 MCQ Sample Paper 2
Question 1:
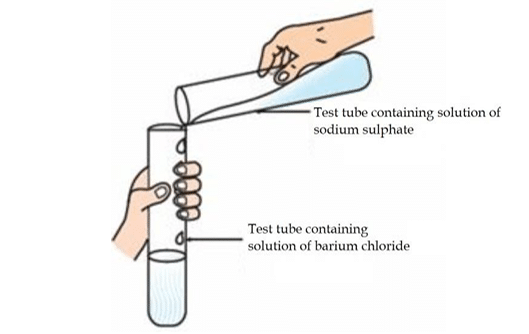
Identify the product which represents the solid state in the above reaction.
a. Barium chloride
b. Barium sulphate
c. Sodium chloride
d. Sodium sulphate
Question 2: The colour of the solution observed after 30 minutes of placing zinc metal to copper sulphate solution is
a. Blue
b. Colourless
c. Dirty green
d. Reddish Brown
Question 3: Mild non-corrosive basic salt is
a. Ca(OH)₂
b. NaCl
c. NaOH
d. NaHCO₃
Question 4: On adding dilute sulphuric acid to a test tube containing a metal ‘X’, a colourless gas is produced when a burning match stick is brought near it. Which of the following correctly represents metal ‘X’?
a. Sodium
b. Sulphur
c. Copper
d. Silver
Question 5: Which one of the following correctly represents Sodium oxide?
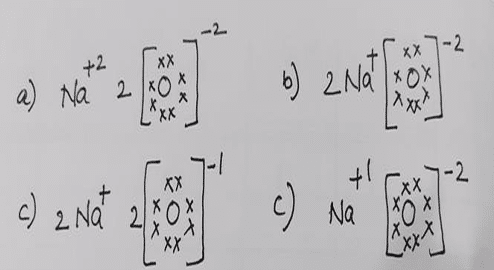
Question 6: An element with atomic number_____ will form a basic oxide.
a. 7 (2,5)
b. 17 (2,8,7)
c. 14 (2,8,4)
d. 11 (2,8,1)
Question 7: An element ‘M’ has 50% of the electrons filled in the 3rd shell as in the 2nd shell. The atomic number of ‘M’ is:
a. 10
b. 12
c. 14
d. 18
Question 8: Generally food is broken and absorbed within the body of organisms. In which of the following organisms is it done outside the body?
a. Amoeba
b. Mushroom
c. Paramoecium
d. Lice
Question 9: Receptors are usually located in sense organs. Gustatory receptors are present in
a. tongue
b. nose
c. eye
d. ear
Question 10: A farmer wants to grow banana plants genetically similar enough to the plants already available in his field. Which one of the following methods would you suggest for this purpose?
a. Regeneration
b. Budding
c. Vegetative propagation
d. Sexual reproduction
Question 11: Height of a plant is regulated by:
a. DNA which is directly influenced by growth hormone.
b. Genes which regulate the proteins directly.
c. Growth hormones under the influence of the enzymes coded by a gene.
d. Growth hormones directly under the influence a gene.
Question 12: A sportsman, after a long break of his routine exercise, suffered muscular cramps during a heavy exercise session. This happened due to:
a. lack of carbon dioxide and formation of pyruvate.
b. presence of oxygen and formation of ethanol.
c. lack of oxygen and formation of lactic acid.
d. lack of oxygen and formation of carbon dioxide.
Question 13: An object is placed in front of a convex mirror. Its image is formed :
a. at a distance equal to the object distance in front of the mirror.
b. at twice the distance of the object in front of the mirror.
c. half the distance of the object in front of the mirror.
d. behind the mirror and it’s position varies according to the object distance.
Question 14: When light enters the atmosphere it strikes on extremely fine particles, which deflect the rays of light in all possible directions, This is due to –
a. reflection of light
b. atmospheric refraction
c. scattering of light
d. dispersion of light
Question 15: In 1987, an agreement was formulated by the United Nations Environment Programme (UNEP) to freeze the production of “X” to prevent depletion of “Y”. “X” and “Y” respectively referred here are:
a. Ozone; CFCs
b. CFCs; rays UV
c. CFCs; Ozone
d. UV rays; Diatomic oxygen
Question 16: Which of the following features relates to biodegradable substances?
a. Broken down by biological processes
b. Remain inert
c. Persist in environment for long time
d. May harm the ecosystem
Question 17: Assertion: Rusting of Iron is endothermic in nature.
Reason: As the reaction is slow, the release of heat is barely evident.
a. Both A and R are true, and R is the correct explanation of A.
b. Both A and R are true, and R is not the correct explanation of A.
c. A is true but R is false.
d. A is false but R is true.
Question 18: Assertion: Probability of survival of an organism produced through sexual reproduction is more than that of organism produced through asexual mode.
Reason: Variations provide advantages to individuals for survival.
a. Both A and R are true, and R is the correct explanation of A.
b. Both A and R are true, and R is not the correct explanation of A.
c. A is true but R is false.
d. A is false but R is true.
Question 19: Assertion : A compass needle is placed near a current carrying wire. The deflection of the compass needle decreases when the magnitude of the current in the wire is increased.
Reason : The strength of a magnetic field at a point near the conductor increases on increasing the current.
a. Both A and R are true, and R is the correct explanation of A.
b. Both A and R are true, and R is not the correct explanation of A.
c. A is true but R is false.
d. A is false but R is true.
Question 20: Assertion: Biodegradable substances result in the formation of compost and natural replenishment.
Reason: It is due to breakdown of complex inorganic substances into simple organic substances.
a. Both A and R are true, and R is the correct explanation of A.
b. Both A and R are true, and R is not the correct explanation of A.
c. A is true but R is false.
d. A is false but R is true.
Question 21: The change in colour of the moist litmus paper in the given set up is due to
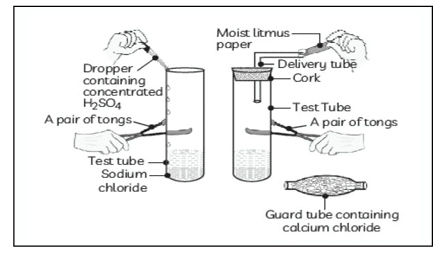
i. presence of acid
ii. presence of base
iii. presence of H⁺(aq) in the solution
iv. presence of Litmus which acts as an indicator
a. i and ii
b. Only ii
c. Only iii
d. Only iv.
Question 22: In the redox reaction
MnO2 + 4HCl → MnCl2 + 2H2 O + Cl2
a. MnO2 is reduced to MnCl2 & HCl is oxidized to H2O
b. MnO2 is reduced to MnCl2 & HCl is oxidized to Cl2
c. MnO2 is oxidized to MnCl2 & HCl is reduced to Cl2
d. MnO2 is oxidized to MnCl2 & HCl is reduced to H2O
Question 23:
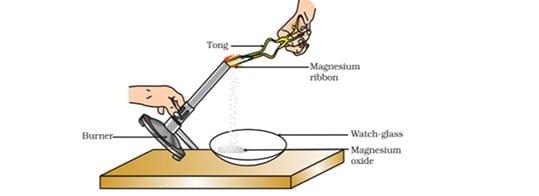
Which of the following is the correct observation of the reaction shown in the above set up?
a. Brown powder of Magnesium oxide is formed.
b. Colourless gas which turns lime water milky is evolved.
c. Magnesium ribbon burns with brilliant white light.
d. Reddish brown gas with a smell of burning Sulphur has evolved.
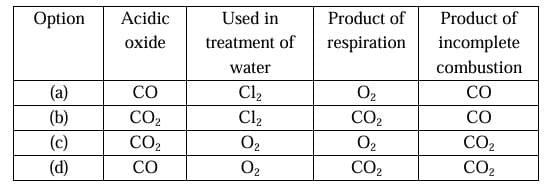
Question 24: With the reference to four gases CO2 ,CO, Cl2 and O2, which one of the options in the table is correct?
Question 25: On placing a copper coin in a test tube containing green ferrous sulphate solution, it will be observed that the ferrous sulphate solution
a. turns blue, and a grey substance is deposited on the copper coin.
b. turns colourless and a grey substance is deposited on the copper coin.
c. turns colourless and a reddish–brown substance is deposited on the copper coin.
d. remains green with no change in the copper coin.
Question 26: Anita added a drop each of diluted acetic acid and diluted hydrochloric acid on pH paper and compared the colors. Which of the following is the correct conclusion?
a. pH of acetic acid is more than that of hydrochloric acid.
b. pH of acetic acid is less than that of hydrochloric acid.
c. Acetic acid dissociates completely in aqueous solution.
d. Acetic acid is a strong acid
Question 27: The formulae of four organic compounds are shown below. Choose the correct option
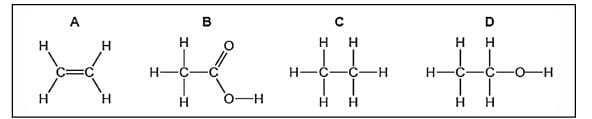
a. A and B are unsaturated hydrocarbons
b. C and D are saturated hydrocarbons
c. Addition of hydrogen in presence of catalyst changes A to C
d. Addition of potassium permanganate changes B to D
Question 28: In the given transverse section of the leaf identify the layer of cells where maximum photosynthesis occurs.
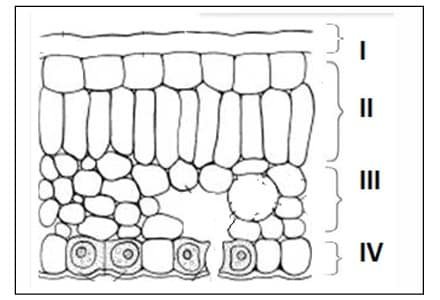
a. I, II
b. II, III
c. III, IV
d. I, IV
Question 29: Observe the experimental setup shown below. Name the chemical indicated as ‘X’ that can absorb the gas which is evolved as a byproduct of respiration.
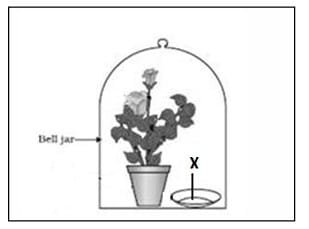
a. NaOH
b. KOH
c. Ca (OH)2
d. K2CO3
Question 30: If a tall pea plant is crossed with a pure dwarf pea plant then, what percentage of F1 and F2 generation respectively will be tall?
a. 25%, 25%
b. 50%, 50%
c. 75%,100%
d. 100%, 75%
Question 31: Observe the three figures given below. Which of the following depicts tropic movements appropriately?
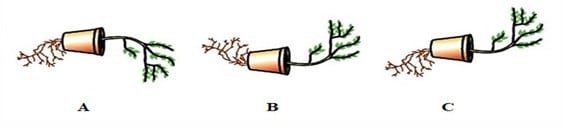
a. B and C
b. A and C
c. B only
d. C only
Question 32: The diagram shown below depicts pollination. Choose the options that will show a maximum variation in the offspring.
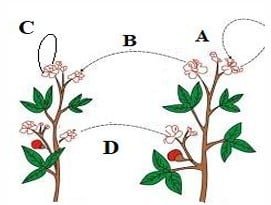
a. A, B and C
b. B and D
c. B, C and D
d. A and C
Question 33: A complete circuit is left on for several minutes, causing the connecting copper wire to become hot. As the temperature of the wire increases, the electrical resistance of the wire
a. decreases.
b. remains the same.
c. increases.
d. increases for some time and then decreases .
Question 34: A copper wire is held between the poles of a magnet.
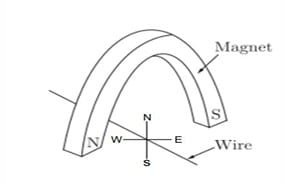
The current in the wire can be reversed. The pole of the magnet can also be changed over. In how many of the four directions shown can the force act on the wire?
a. 1
b. 2
c. 3
d. 4
Question 35:
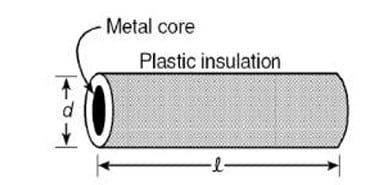
Plastic insulation surrounds a wire having diameter d and length l as shown above. A decrease in the resistance of the wire would be produced by an increase in the
a. length l of the wire
b. diameter d of the wire
c. temperature of the wire
d. thickness of the plastic insulation
Question 36: Which of the following pattern correctly describes the magnetic field around a long straight wire carrying current?
a. straight lines perpendicular to the wire.
b. straight lines parallel to the wire.
c. radial lines originating from the wire.
d. concentric circles centred around the wire.
Question 37: Assertion: Silver bromide decomposition is used in black and white photograph.
Reason: Light provides energy for this exothermic reaction.
a. Both A and R are true, and R is the correct explanation of A.
b. Both A and R are true, and R is not the correct explanation of A.
c. A is true but R is false.
d. A is false but R is true.
Question 38: Assertion: Height in pea plants is controlled by efficiency of enzymes and is thus genetically controlled.
Reason: Cellular DNA is the information source for making proteins in the cell.
a. Both A and R are true, and R is the correct explanation of A.
b. Both A and R are true, and R is not the correct explanation of A.
c. A is true but R is false.
d. A is false but R is true.
Question 39: Assertion: Amphibians can tolerate mixing of oxygenated and deoxygenated blood.
Reason: Amphibians are animals with two chambered heart
a. Both A and R are true, and R is the correct explanation of A.
b. Both A and R are true, and R is not the correct explanation of A.
c. A is true but R is false.
d. A is false but R is true.
Question 40: Assertion: On freely suspending a current – carrying solenoid, it comes to rest in Geographical N-S direction.
Reason : One end of current carrying straight solenoid behaves as a North pole and the other end as a South pole, just like a bar magnet.
a. Both A and R are true, and R is the correct explanation of A.
b. Both A and R are true, and R is not the correct explanation of A.
c. A is true but R is false.
d. A is false but R is true.
Disclaimer: We have provide you with the accurate handout of “Science Class 10 MCQ Sample Paper 2“. If you feel that there is any error or mistake, please contact me at anuraganand2017@gmail.com. The above CBSE study material present on our websites is for education purpose, not our copyrights.
All the above content and Screenshot are taken from Science Class 10 NCERT Textbook, CBSE Sample Paper, CBSE Old Sample Paper, CBSE Board Paper and CBSE Support Material which is present in CBSEACADEMIC website, NCERT website This Textbook and Support Material are legally copyright by Central Board of Secondary Education or NCERT. We are only providing a medium and helping the students to improve the performances in the examination.
Images and content shown above are the property of individual organisations and are used here for reference purposes only. To make it easy to understand, some of the content and images are generated by AI and cross-checked by the teachers. For more information, refer to the official CBSE textbooks available at cbseacademic.nic.in.
