Matter in our Surroundings Class 9 Notes – This CBSE note for class 9 science chapter 1 notes provides a summary and revision of the topic “Matter in Our Surroundings.” These notes are helpful resource for class 9 students who want prepare for exams. It is recommended to also refer to NCERT solutions for a better understanding of the chapter.
Matter in our Surroundings Class 9 Notes
What is Matter?
Any substance that occupies space and has mass is called matter. Matter is a physical material that has mass and volume and that occupies space. Matter is made up of very tiny particles called atoms. Some of the examples of matter are sand, sugar, air, oxygen, water, etc. These forms can be changed by altering temperature and pressure. Matter can exist in different forms, like solid, liquid, and gas.
Characteristics of Matter
- Matter is made up of very tiny particles.
- Matter occupies space and also has mass.
- Particles of matter have space between them and are in constant motion.
- The attraction between particles of matter exists.
- Matter is not continuous but particulate.
- Matter can be in different forms (solid, liquid, or gas).
- These forms of matter can change based on changes in temperature and pressure.
States of Matter
Matter around us exists in three different states– solid, liquid and gas. These states of matter arise due to the variation in the characteristics of the particles of matter.
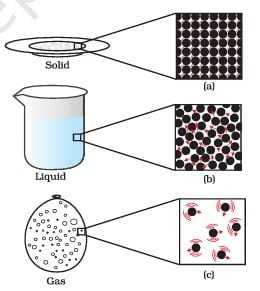
The process of changing the state of matter is known as phase transition. Temperature and pressure variations are what cause the condition to shift. For example, when a solid is heated, it will melt and turn into a liquid, and if the temperature is raised, the liquid will boil and turn into a gas. Similarly, cooling a gas can condense it into a liquid and then freeze it into a solid.
Summarizing properties of solids, liquids, and gases
| Property | Solids | Liquids | Gases |
|---|---|---|---|
| Shape | Definite and fixed | Take the shape of container | Fill container completely |
| Volume | Definite and fixed | Take the volume of container | Fill container completely |
| Particle arrangement | Particles are closely packed and in a fixed arrangement | Particles are close but not as closely packed as solids and move around each other | Particles are far apart and move randomly |
| Particle mobility | Low | More than solids, less than gases | High |
| Compressibility | Not easily compressed | Compressed slightly | Easily compressed |
| Energy | less energy | Medium | Heigh |
Characteristics of Particles of Matter
- Size: The size of particles can vary; some of the particles are smaller than atoms, and others are as large as molecules.
- Arrangement: The arrangement of particles in a substance can be random, ordered, or intermediate.
- Motion: Due to kinetic energy, particles in matter are in constant motion, exhibiting random movement.
- Interparticle Forces: A substance’s condition and qualities are affected by the forces of attraction or repulsion experienced by its particles.
- Energy: The particles in a substance can possess different forms of energy, such as kinetic (motion), potential (stored), and thermal (heat).
- Charge: The electrical charge of a particle in matter, which can be positive, negative, or neutral, can have an impact on how that particle behaves and interacts with other particles.
What is Diffusion?
Diffusion is the movement of particles in a substance from an area of higher concentration to an area of lower concentration until the concentration is evenly distributed.
- Diffusion occurs due to the random motion of particles and the interparticle forces that cause particles to move into regions with lower particle density.
- The rate of diffusion can be influenced by factors such as temperature, pressure, and the size and type of the particles.
- Diffusion is an important process in various fields, including chemistry, biology, and materials science.
Can Matter Change Its State?
Yes, matter can change its state. There are three possible states for matter: solid, liquid, and gas. The state of a substance is determined by its temperature and pressure, and a change in either of these two variables can cause a substance to change its state. For example, heating ice (a solid) will cause it to melt and become liquid water. On the other hand, cooling will cause liquid water to freeze and solidify into ice. This change of state is called phase transition, and it is a common physical phenomenon that occurs in matter.
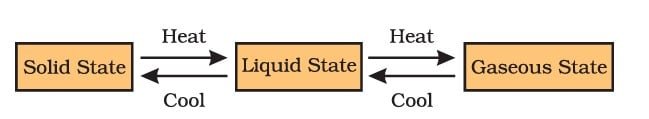
Melting point—The temperature at which a solid changes into a liquid at normal atmospheric pressure is referred to as its melting point.
Boiling point—The temperature at which a liquid begins to change into a gas at normal atmospheric pressure is called its boiling point.
Latent heat of fusion—The latent heat of fusion of a solid refers to the amount of heat energy needed to turn 1 kilogram of the solid into a liquid at its atmospheric pressure.
Latent heat of vaporization—A liquid’s latent heat of vaporization is the amount of heat energy required to convert 1 kilogram of the liquid into a gas at standard atmospheric pressure at its boiling point.
Evaporation—It’s a natural and surface phenomenon. When a liquid reaches a temperature below its boiling point, its evaporation helps it to transform into a gas. Evaporation only takes place on a liquid’s surface.
Factors affecting evaporation
- Temperature: Temperature causes an increase in evaporation rate.
- Surface area: Evaporation happens more quickly the larger the surface area.
- Humidity: Humidity has an inverse relationship with evaporation rate.
- Airflow: The stronger the airflow, the quicker the evaporation.
- Pressure: The lower the pressure, the faster the evaporation process takes place.
- Type of liquid: Due to variations in their vapor pressure and intermolecular interactions, different liquids evaporate at varying rates. For example, evaporation is easy for liquids like acetone, which needs only the body temperature that is 37o C.
Some measurable quantities and their units
| Quantity | Unit |
|---|---|
| Mass | Kilogram (kg) |
| Length | Meter (m) |
| Time | Second (s) |
| Temperature | Kelvin (K) |
| Volume | Cubic meter (m^3) |
| Density | Kilogram per cubic meter (kg/m^3) |
| Pressure | Pascal (Pa) |
| Energy | Joule (J) |
| Heat | Joule (J) |
| Force | Newton (N) |
Plasma State
Plasma refers to a state of matter that contains highly energetic and charged particles in the form of ionized gases. Examples of plasma include the gases found inside fluorescent lights and neon signs. When electrical energy is applied, the gases become charged, producing a glowing plasma within the light or sign. The color of the glow depends on the type of gas, and plasma is also found in stars, where it is created by extremely high temperatures.
Bose-Einstein Condensate
Satyendra Nath Bose, an Indian physicist, initially proposed the concept of Bose-Einstein condensate, which Albert Einstein later developed. Eric A. Cornell, Wolfgang Ketterle, and Carl E. Wieman, who shared the 2001 Nobel Prize in Physics for their work, were responsible for its successful development. When a gas is cooled to extraordinarily low temperatures, it acquires a density that is significantly lower than that of ordinary air, creating the Bose-Einstein condensate. Bose-Einstein condensation is the name given to this process.
Disclaimer: We have taken an effort to provide you with the accurate handout of “Matter in our Surroundings Class 9 Notes“. If you feel that there is any error or mistake, please contact me at anuraganand2017@gmail.com.
The above CBSE study material present on our websites is for education purpose, not our copyrights. All the above content and Screenshot are taken from Science Class 9 NCERT Textbook, CBSE Sample Paper, CBSE Old Sample Paper, CBSE Board Paper and CBSE Support Material which is present in CBSEACADEMIC website, NCERT websiteThis Textbook and Support Material are legally copyright by Central Board of Secondary Education. We are only providing a medium and helping the students to improve the performances in the examination.
Images and content shown above are the property of individual organizations and are used here for reference purposes only.
For more information, refer to the official CBSE textbooks available at cbseacademic.nic.in
