Class 10 Science Chapter 4, Carbon and Its Compounds, focuses on the unique bonding properties of carbon that make it an essential element in organic chemistry. In this chapter, students learn about covalent bonding, versatile carbon chains, functional groups, and chemical reactions like combustion, oxidation, addition, and substitution.
Science Class 10 Chapter 4 NCERT Solutions
1. What would be the electron dot structure of carbon dioxide, which has the formula CO2?
Answer: Carbon dioxide is made of 1 carbon and 2 oxygen atoms. Carbon shares 4 electrons – 2 with each oxygen. Each oxygen shares two electrons with carbon. This makes double bonds between carbon and oxygen.
..
:O::C::O:
..
or
O = C = O
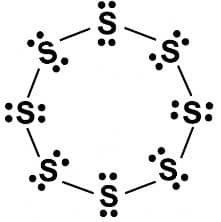
2. What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur? (Hint – The eight atoms of sulphur are joined together in the form of a ring.)
Answer: Sulphur atoms form a ring of 8 atoms. Each sulphur shares 1 electron with its neighbour. The rest of the electrons stay as dots around each atom.
3. How many structural isomers can you draw for pentane?
Answer: Pentane has 5 carbon atoms. It can be arranged in 3 different ways –
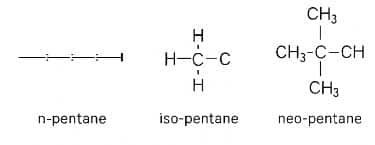
- Straight line (n-pentane)
- One branch (isopentane)
- Central carbon with 4 branches (neopentane)
4. What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
Answer: The two properties of carbon that make many compounds are:
- Catenation: Carbon can join with other carbon atoms to make long chains.
- Tetravalency: Carbon can make 4 bonds with other atoms like hydrogen, oxygen, etc.
5. What will be the formula and electron dot structure of cyclopentane?
Answer:
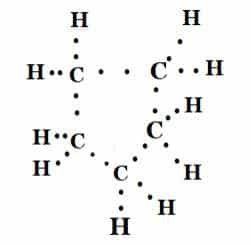
The formula is C5H10
Structure: 5 carbon atoms in a ring, each with hydrogen atoms.
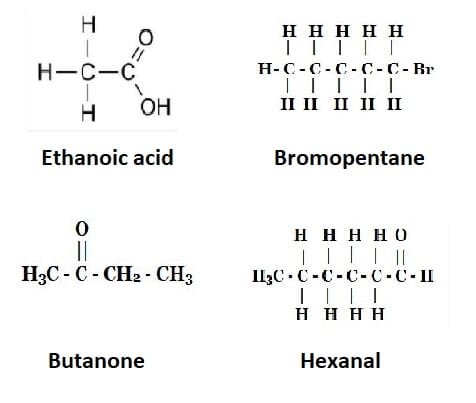
6. Draw the structures for the following compounds.
(i) Ethanoic acid
(ii) Bromopentane*
(iii) Butanone
(iv) Hexanal.
*Are structural isomers possible for bromopentane?
Answer: The structures of the above compounds are:
- Ethanoic acid: 2 carbon atoms, one with a COOH group (acid)
- Bromopentane: 5 carbon atoms, one has a bromine (Br) attached.
- Butanone: 4 carbon atoms, with a ketone group (=O) on the second carbon
- Hexanal: 6 carbon atoms, with an aldehyde group (-CHO) at the end
Yes! Bromine can attach to different carbon atoms:
7. How would you name the following compounds?
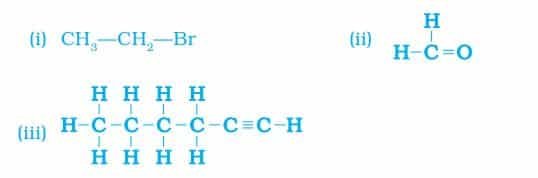
Answer:
- Bromoethane
- Methanal or Formaldehyde
- 1 – Hexyne
8. Why is the conversion of ethanol to ethanoic acid an oxidation reaction?
Answer: Ethanol (CH3CH2OH) is converted to ethanoic acid (CH3COOH). The -OH group in ethanol is oxidised to a -COOH group. This involves the addition of oxygen or removal of hydrogen, which is the hallmark of oxidation. Oxidising agents like acidified potassium dichromate or alkaline KMnO4 are used.
9. A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
Answer: Oxygen + ethyne burns completely and gives a very hot blue flame and helps to melt metal. Air + ethyne burns incompletely; because of this, it gives a sooty flame and produces less heat. Welding requires high temperatures; that’s why oxygen is used.
10. How would you distinguish experimentally between an alcohol and a carboxylic acid?
Answer: To test alcohol vs carboxylic acid, add baking soda (NaHCO3). Carboxylic acid will produce CO₂ gas. Alcohol will show no reaction. You can also use litmus paper; carboxylic acid turns blue litmus red, which means acidic. Alcohols are neutral, and they will not change colour.
11. What are oxidising agents?
Answer: Substances that add oxygen or remove hydrogen from other compounds. They cause oxidation in other substances while getting reduced themselves. For example, potassium permanganate (KMnO₄), potassium dichromate (K2Cr2O7) and hydrogen peroxide (H2O2)
12. Would you be able to check if water is hard by using a detergent?
Answer: No, detergent works in both hard and soft water. Instead of detergent, we can use soap. Hard water + soap = scum, no foam. Soft water + soap = foam. That’s why soap is better for testing water hardness.
13. People use a variety of methods to wash clothes. Usually after adding the soap, they ‘beat’ the clothes on a stone or beat them with a paddle, scrub with a brush, or agitate the mixture in a washing machine. Why is agitation necessary to get clean clothes?
Answer: ‘Agitation’ means scrubbing, and beating, and shaking helps to remove dirt from cloth; soap breaks the oil into tiny pieces. But the dirt is still stuck inside the cloth. So, we beat, scrub or shake the clothes to pull the dirt out.
14. Ethane, with the molecular formula C₂H₆, has
(a) 6 covalent bonds.
(b) 7 covalent bonds.
(c) 8 covalent bonds.
(d) 9 covalent bonds.
Answer: (b) 7 covalent bonds.
15. Butanone is a four-carbon compound with the functional group
(a) carboxylic acid.
(b) aldehyde.
(c) ketone.
(d) alcohol.
Answer: (c) ketone.
16. While cooking, if the bottom of the vessel is getting blackened on the outside, it means that
(a) The food is not cooked completely.
(b) The fuel is not burning completely.
(c) The fuel is wet.
(d) The fuel is burning completely.
Answer: (b) the fuel is not burning completely.
17. Explain the nature of the covalent bond using the bond formation in CH₂Cl.
Answer: Carbon has 4 valence electrons and needs 4 more to complete its octet. All atoms share electrons, forming covalent bonds. Chlorine also shares 1 electron and keeps 6 others as lone pairs. This sharing makes the molecule stable without gaining or losing electrons.
18. Draw the electron dot structures for
(a) ethanoic acid.
(b) H₂S.
(c) propanone.
(d) F2.
Answer:
- (a) ethanoic acid: Shows two carbon atoms, one with a carboxylic acid group (–COOH), with shared and lone electrons.
- (b) H₂S: Sulphur shares electrons with two hydrogen atoms and has two lone pairs.
- (c) propanone: Three carbon atoms, with a ketone group (=O) on the middle carbon.
- (d) F₂: Two fluorine atoms share one pair of electrons, and each has three lone pairs.
19. What is a homologous series? Explain with an example.
Answer: When carbon atoms can be linked together to form chains of varying lengths. These chains can be branched also. In addition, hydrogen atoms or other atoms on these carbon chains can be replaced by any of the functional groups that we saw above. The presence of a functional group such as alcohol decides the properties of the carbon compound, regardless of the length of the carbon chain. For example, the chemical properties of CH3OH, C2H5OH, C3H7OH and C4H9OH are all very similar. Hence, such a series of compounds in which the same functional group substitutes for hydrogen in a carbon chain is called a homologous series.
20. How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties?
Answer: Ethanol and ethanoic acid look similar, but they have different properties. Ethanol is an alcohol with a sweet smell and is basically used in sanitisers and alcoholic drinks. Ethanoic acid is also known as acetic acid, smells like vinegar, and is used for cooking and cleaning. Chemically ethanol does not change blue litmus paper, but ethanoic acid turns it red because it is acidic. When ethanol reacts with sodium metal, it produces hydrogen gas slowly; on the other hand, ethanoic acid reacts more vigorously, releasing bubbles quickly.
21. Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?
Answer: Most dirt is oily in nature, and as you know, oil does not dissolve in water. The molecules of soap are sodium or potassium salts of long-chain carboxylic acids. The ionic end of soap interacts with water, while the carbon chain interacts with oil. The soap molecules thus form structures called micelles, where one end of the molecules is towards the oil droplet while the ionic end faces outside. This forms an emulsion in water. The soap micelle thus helps in pulling out the dirt in water, and we can wash our clothes clean.
22. Why are carbon and its compounds used as fuels for most applications?
Answer: Carbon and its compounds are used as fuels because they are rich in energy and burn easily in the air. When the carbon compounds like coal, petrol, diesel or natural gas burn, they combine with oxygen and release a lot of heat. This heat is used for cooking, transport, electricity and many other applications. Also, carbon compounds are easily available, cheap and can be stored and transported.
23. Explain the formation of scum when hard water is treated with soap.
Answer: When soap is added to hard water, it forms a white, sticky substance called scum. This happens because hard water contains calcium (Ca2+) and magnesium (Mg2+) ions. These ions react with the soap and form insoluble salts. These salts do not dissolve in water and appear as scum. This scum reduces the cleaning power of soap and wastes it. That’s why soap does not work well in hard water.
24. What change will you observe if you test soap with litmus paper (red and blue)?
Answer: Soap is basic in nature. When you test it with litmus paper:
- Red litmus paper turns blue.
- Blue litmus paper stays blue.
This shows that soap has a basic (alkaline) pH.
25. What is hydrogenation? What is its industrial application?
Answer: Hydrogenation is a chemical process in which hydrogen gas (H₂) is added to unsaturated compounds (like oils) in the presence of a catalyst. This converts unsaturated fats into saturated fats. In industry, hydrogenation is widely used in the food industry to convert vegetable oils into solid fats, like vanaspati ghee. This makes the oil more stable and gives it a longer shelf life.
26. Which of the following hydrocarbons undergo addition reactions?
C2H6, C3H8, C3H6, C2H2 and CH4
Answer:
- C₂H₆ (ethane) – saturated → no addition reaction
- C₃H₈ (propane) – saturated → no addition reaction
- C₃H₆ (propene) – unsaturated (double bond) → undergoes addition reaction
- C₂H₂ (ethyne) – unsaturated (triple bond) → undergoes addition reaction
- CH₄ (methane) – saturated → no addition reaction
27. Give a test that can be used to differentiate between saturated and unsaturated hydrocarbons.
Answer: A simple test to differentiate between saturated and unsaturated hydrocarbons is the bromine water test.
- Unsaturated hydrocarbons decolourise bromine water. This happens because they have double or triple bonds that react with bromine.
- Saturated hydrocarbons do not react with bromine water, so the reddish-brown colour stays.
28. Explain the mechanism of the cleaning action of soaps.
Answer: Soap cleans by surrounding oily dirt with special molecules. Each soap molecule has two ends: one that sticks to water and one that sticks to oil. When you mix soap with water and rub it on dirty clothes, the soap forms tiny balls called micelles that trap the dirt. When you rinse, water carries these micelles away, so the dirt goes too.
Disclaimer: We have provide you with the accurate handout of “Science Class 10 Chapter 4 NCERT Solutions“. If you feel that there is any error or mistake, please contact me at anuraganand2017@gmail.com. The above CBSE study material present on our websites is for education purpose, not our copyrights.
All the above content and Screenshot are taken from Science Class 10 NCERT Textbook, CBSE Sample Paper, CBSE Old Sample Paper, CBSE Board Paper and CBSE Support Material which is present in CBSEACADEMIC website, NCERT website This Textbook and Support Material are legally copyright by Central Board of Secondary Education or NCERT. We are only providing a medium and helping the students to improve the performances in the examination.
Images and content shown above are the property of individual organisations and are used here for reference purposes only. To make it easy to understand, some of the content and images are generated by AI and cross-checked by the teachers. For more information, refer to the official CBSE textbooks available at cbseacademic.nic.in.

Question numbering error is there as 10 is repeated twice
Thank you…